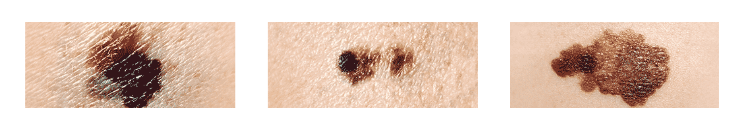
Melanoma is a type of skin cancer that begins in the cells that give the skin its color, known as melanocytes. Initial signs of a possible case of melanoma are unusual moles, sores, blemishes, lumps, or changes in the look or feel of an area of skin.
To help identify melanoma, you can follow the ABCDE system of characteristics.
Asymmetry
One half of the mole is unlike the other.
Border
The border of the mole is irregular, scalloped, or poorly defined.
Color
The color of the mole varies from one area to the next and can exhibit shades of tan, brown or black, white, red, or blue.
Diameter
Melanomas are usually larger than 6 millimeters (about the size of a pencil eraser) when diagnosed, but they can be smaller.
Evolving
The mole looks different than others on your body or is changing in size, shape, or color.